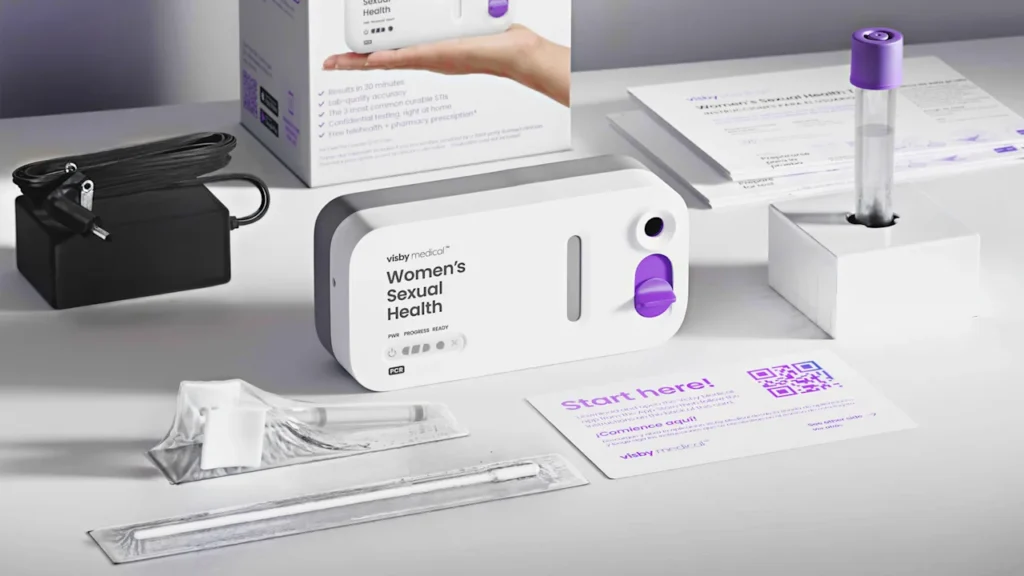
The worst part of any medical is waiting for results. That can be especially true of sexual health tests. Those conducted in person can take 24–48 hours, but if they’re submitted via at-home collection kit and mailed to a lab, it can take even longer.
A new test from diagnostics company Visby Medical, launching nationwide today is changing that. Now that it’s successfully completed a pilot period, the company is bringing a 30-minute, lab-accurate PCR test for three common sexually transmitted infections to women at home. From a self-collected vaginal swab, the $149.99 test can diagnose chlamydia, gonorrhea, and trichomoniasis—three common infections that can all easily be treated with antibiotics. It also connects patients who test positive with a healthcare provider via United Healthcare’s OptumNow telehealth service.
“If you tell somebody, ‘I have an STD test for the home,’ what they think I mean is ‘You’re going to give me a collection kit, I’m going to pee in the cup and send it back and get a result a week later,’” says Adam de la Zerda, founder and CEO of Visby Medical. With his machine, he says, “all they have to do is close the lid and the test starts running, and in 30 minutes you’ll get your result.”
Cleared by the FDA in March as the first at-home diagnostic for these STIs, Visby’s test is now going wide with the test following a smaller pilot launch on its site and via diagnostics platform Everlywell. It parlayed that approval into a funding round that in July had brought in $55 million, led by healthcare investment firm Catalio Capital Management.
The company last raised money in 2022, when a $135 million funding round—focused on developing the STI test and a point-of-care COVID and flu test—valued the company at more than $1 billion. According to PitchBook, it’s raised a total of $486 million

To underscore the convenience element of the new test, Visby is also making it available for same-day delivery via GoPuff and DoorDash in 10 major cities including Las Vegas, Atlanta, New York, San Francisco, and Seattle.
Orders can be placed via the Visby website, which helps identify the best delivery option for a buyer, and dispatched via one of the apps. “It looks and feels just like ordering lunch,” de la Zerda says. “It’s nobody’s business what you just received from DoorDash—it could be a bag of burritos or a Visby test.”
Visby’s test is also getting a cosign from a competitor, with digital health platform Everlywell selling it.
From test to treatment
De la Zerda says that the STI test has been the company’s goal since it was founded in 2012. Visby had just finished its initial clinical trials on a test for use in doctor’s offices when the pandemic forced a pivot to COVID, for which Visby developed a point of care test. Even then, the focus was at-home PCR, with Visby landing $19 million from a federal prize competition with an early, point-of-care version of the test.
The FDA authorization—via the agency’s De Novo pathway for novel medical devices—took roughly a year from submission to approval, included Visby’s app, which is powered by Google Cloud to decode the test results.The test itself demonstrated the ability to identify 98.8% of negative and 97.2% of positive chlamydia samples; 99.1% of negative and 100% of positive gonorrhea samples; and 98.5% of negative and 97.8% positive trichomoniasis samples.
Visby’s ability to say that its test is actually diagnosing STIs—as opposed to other at-home STI screenings that require a lab test to confirm their results—meant de la Zerda wanted to build an easy way to be treated into the test process and price tag.
“We got a true diagnostic claim,” de la Zerda says. “That enabled us to go to folks like United Health, OptumNow, and say ‘let’s leverage that telemedicine platform you guys have built and create that connectivity for people.’” With a 24/7 provider network active in all 50 states, OptumNow can connect Visby users with a clinician and have a prescription sent to a local pharmacy within about 7–10 minutes, de la Zerda says.
“It’s a powerful thing to enable somebody to test for a stigmatized condition in the privacy of their home and not just leave them hanging with a diagnosis,” he says. That’s part of how Visby’s test ended up on a competitor’s platform.
Team of rivals
Besides the Visby website and its delivery partners, the test is also being offered via Everlywell, a digital health platform that offers home testing on conditions that include fertility, STIs, food sensitivities, and immune health.
“STI specifically is an incredibly important, undertested epidemic where this [at-home] format lends itself to eliminating stigma and creating privacy,” says Julia Cheek, founder and CEO of Everlywell. Though the company sells its own five-panel sexual health testing kit for, it functions as a blood and urine collection kit that users have to mail into a lab for results. Cheek says the speed and convenience of Visby’s test made it an obvious choice for Everlywell, which serves a user base of 80% women.
“It’s not fully comprehensive yet, but we want to be able to meet people where they are and offer them different options,” she syas. “We fundamentally believe the consumer deserves access to whatever test is available, accurate and gets them what they need.”
Everlywell has offered Visby’s test since August, and she says users have responded positively, with both companies already planning to invest further in marketing the test to Everlywell users in 2026.
Even as the STI test shows promise, de la Zerda sees today’s wide launch as a starting point. “If you rank the top 200 tests that people are running on a PCR machine, just about every single one of them we can have a Visby test to run it as well,” he says. “It’s the same technology.”
